![Catalytic activity of K2Ba[Ni(NO2)6] on the thermolysis and laser ignition of CL-20, FOX-7 and TKX-50 - ScienceDirect Catalytic activity of K2Ba[Ni(NO2)6] on the thermolysis and laser ignition of CL-20, FOX-7 and TKX-50 - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022369721004777-gr2.jpg)
Catalytic activity of K2Ba[Ni(NO2)6] on the thermolysis and laser ignition of CL-20, FOX-7 and TKX-50 - ScienceDirect

Figure 4 from Comparative catalytic properties of Ni(OH)₂ and NiO nanoparticles towards the degradation of nitrite (NO₂¯) and nitric oxide (NO) | Semantic Scholar
Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitr
![Fragmentation of [Ni(NO3)3]−: A Study of Nickel–Oxygen Bonding and Oxidation States in Nickel Oxide Fragments | Inorganic Chemistry Fragmentation of [Ni(NO3)3]−: A Study of Nickel–Oxygen Bonding and Oxidation States in Nickel Oxide Fragments | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.6b00812/asset/images/medium/ic-2016-008124_0005.gif)
Fragmentation of [Ni(NO3)3]−: A Study of Nickel–Oxygen Bonding and Oxidation States in Nickel Oxide Fragments | Inorganic Chemistry

Spectra of the solid complexes spread on filter paper, at 25 °C: [Ni II... | Download Scientific Diagram

Redox Chemistry of Nickel(II) Complexes Supported by a Series of Noninnocent β-Diketiminate Ligands | Inorganic Chemistry

A Novel Organometallic Coordination Polymer as a Heterogeneous Catalyst for C−C Bond Formation - Yang - 2022 - European Journal of Inorganic Chemistry - Wiley Online Library
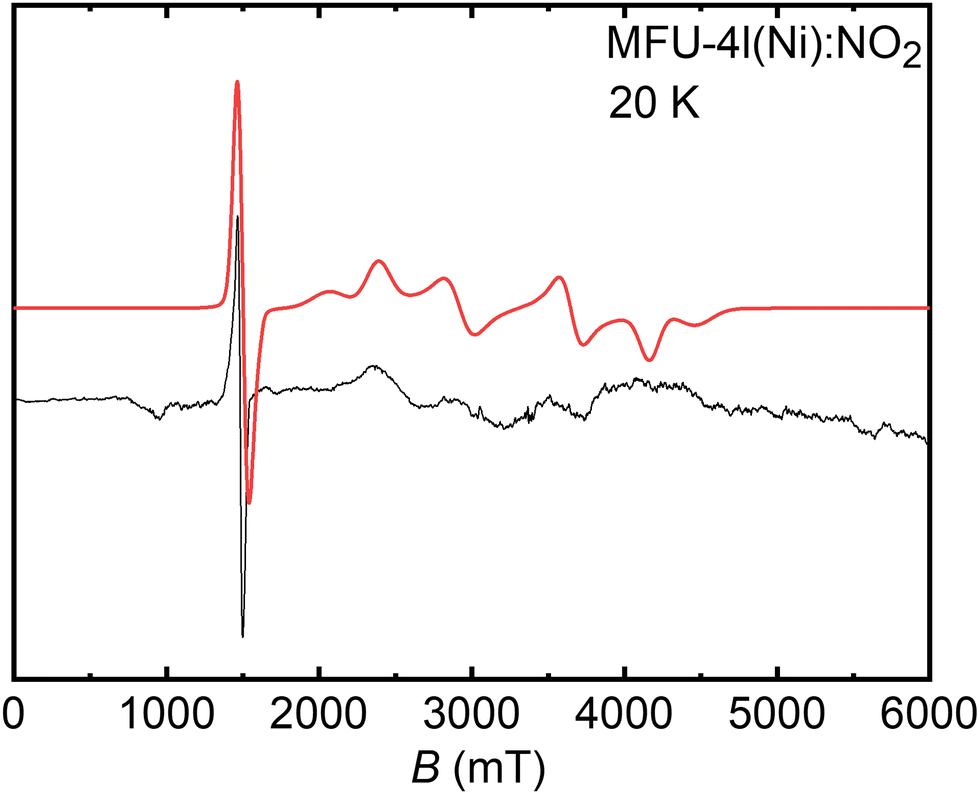
Unveiling the atomistic and electronic structure of Ni II –NO adduct in a MOF-based catalyst by EPR spectroscopy and quantum chemical modelling - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D3CP01449E

Catalysts | Free Full-Text | LLDPE-like Polymers Accessible via Ethylene Homopolymerization Using Nitro-Appended 2-(Arylimino)pyridine-nickel Catalysts
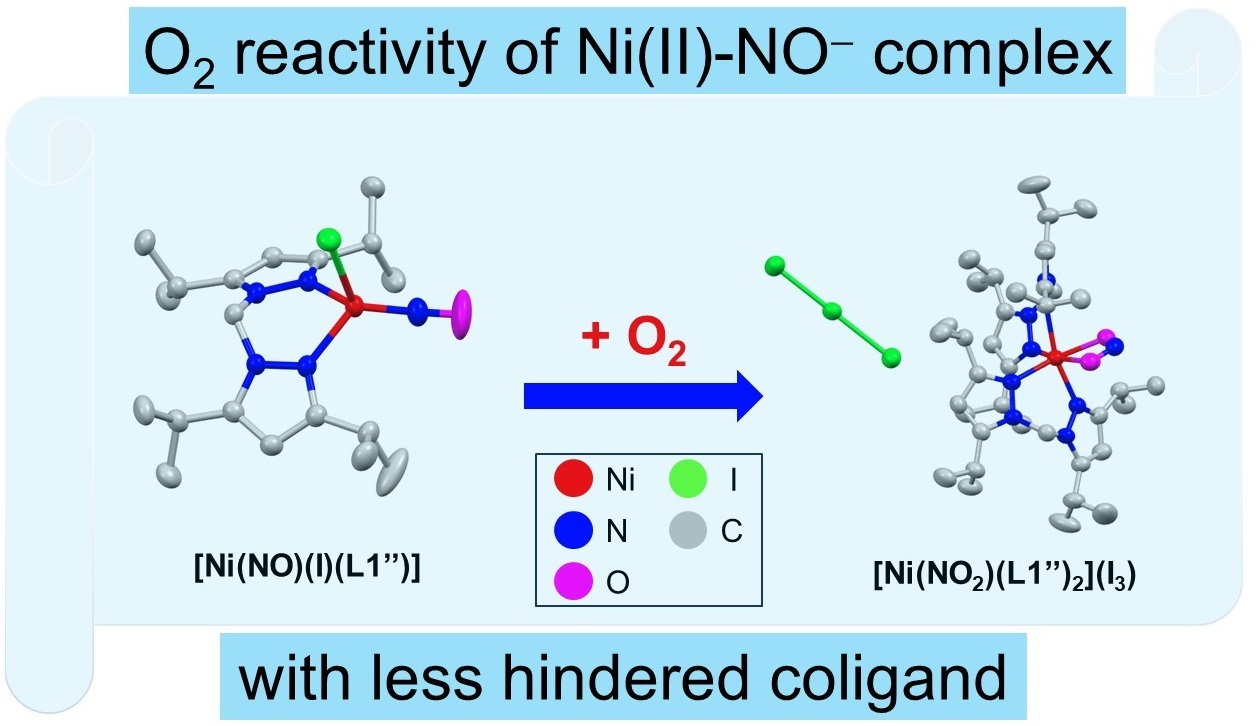
Molecules | Free Full-Text | Coordinatively Unsaturated Nickel Nitroxyl Complex: Structure, Physicochemical Properties, and Reactivity toward Dioxygen

![trans-Bis(nitrito-κN)bis(propane-1,3-diamine)nickel(II) [Ni(C3H10N3)2(NO2)2] : r/chemistry trans-Bis(nitrito-κN)bis(propane-1,3-diamine)nickel(II) [Ni(C3H10N3)2(NO2)2] : r/chemistry](https://preview.redd.it/trans-bis-nitrito-%CE%BAn-bis-propane-1-3-diamine-nickel-ii-ni-v0-b4n0ievj9rea1.jpg?width=632&format=pjpg&auto=webp&s=d3590d49d3c90d80ef86936c014d78a4b4d092d2)