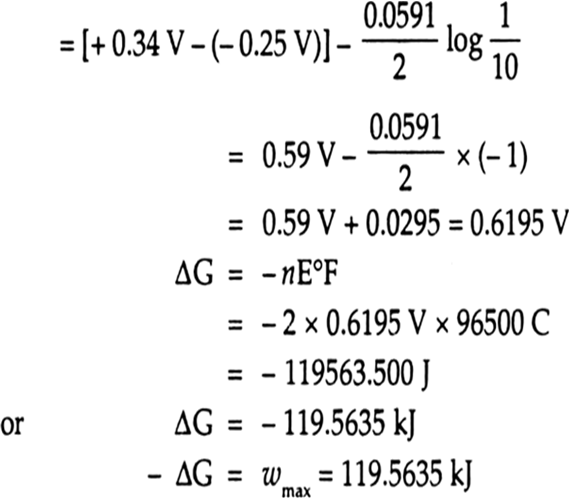Surface Restructuring of Nickel Sulfide Generates Optimally Coordinated Active Sites for Oxygen Reduction Catalysis - ScienceDirect

Heterogeneous histories of Ni‐bearing pyrrhotite and pentlandite grains in the CI chondrites Orgueil and Alais - Berger - 2016 - Meteoritics & Planetary Science - Wiley Online Library

Surface Restructuring of Nickel Sulfide Generates Optimally Coordinated Active Sites for Oxygen Reduction Catalysis - ScienceDirect

4 15. E.M.F. of Ni(s)[Ni2+ (aq) || Cu2+ (aq)|Cu(s) cell can be increased by (1) Adding NH, in the right half-cell (2) Increasing the conc. of Ni2+ ions (3) Adding dimethyl

Surface Activation and Ni‐S Stabilization in NiO/NiS2 for Efficient Oxygen Evolution Reaction - Zhang - 2022 - Angewandte Chemie International Edition - Wiley Online Library
XPS spectra of (Fe,Ni) 3 S 4 : (a) Fe 2p fresh, (b) Fe 2p calcined at... | Download Scientific Diagram

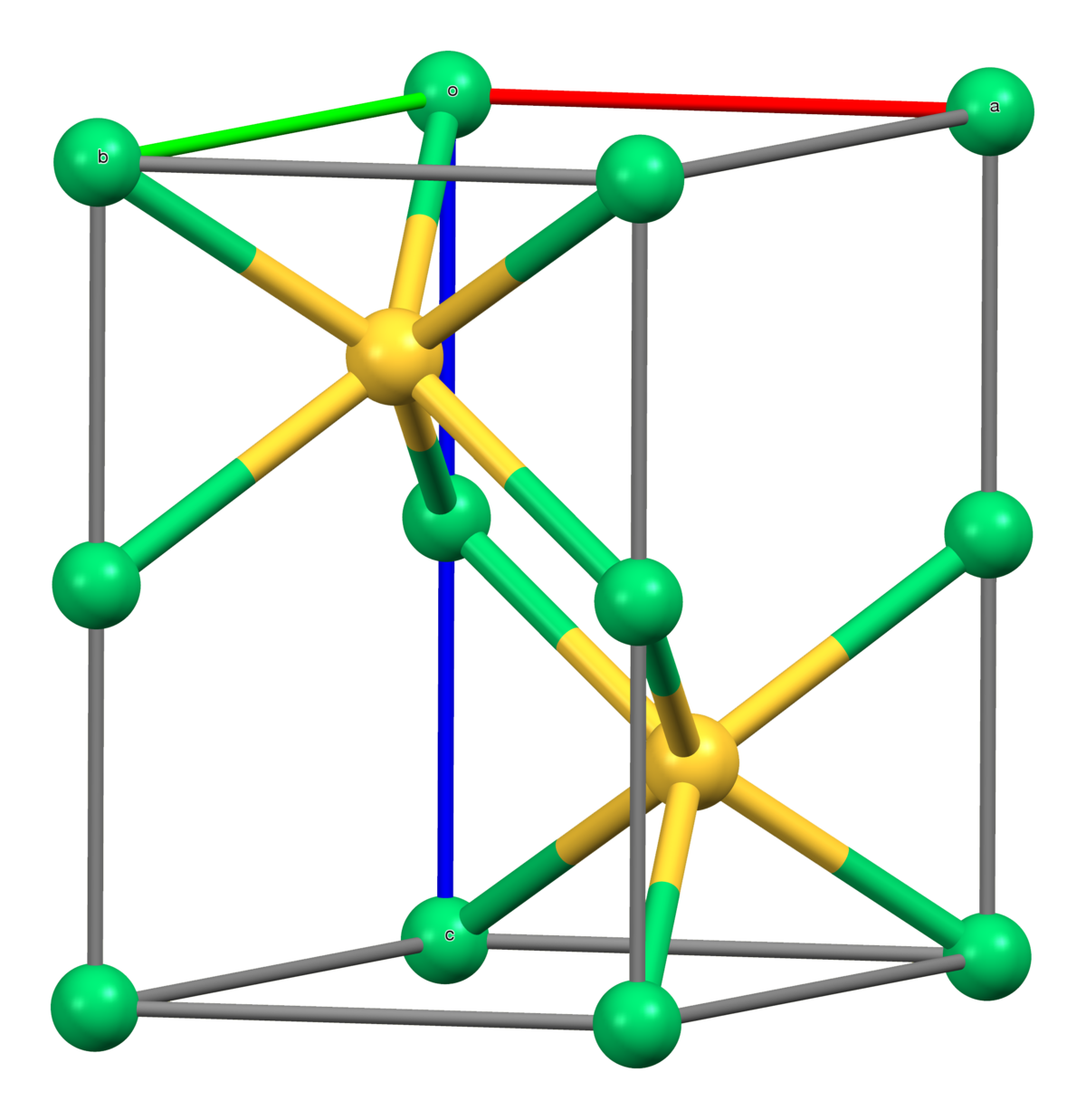
One-Step Synthesis of Nickel Sulfides and Their Electrocatalytic Activities for Hydrogen Evolution Reaction: A Case Study of Crystalline h-NiS and o-Ni9S8 Nanoparticles | ACS Applied Energy Materials
![The Nernst equation the following electrochemical cell will be: Ni(s) | Ni2+ (aq)|| Ag+ (aq)| Ag A) Ecell = Eºcell-RT/F[In[Ni2+]/[Ag+12] B) Ecell = Eccl1-RT/2F[In[Ni2+1/[Ag+1?] C) Ecell = Eºcell-RT/2F[In[Ag+]2/[Ni2+]] D) Ece = Eccl1-RT/2F[In[Ni2+1/[Ag+l] The Nernst equation the following electrochemical cell will be: Ni(s) | Ni2+ (aq)|| Ag+ (aq)| Ag A) Ecell = Eºcell-RT/F[In[Ni2+]/[Ag+12] B) Ecell = Eccl1-RT/2F[In[Ni2+1/[Ag+1?] C) Ecell = Eºcell-RT/2F[In[Ag+]2/[Ni2+]] D) Ece = Eccl1-RT/2F[In[Ni2+1/[Ag+l]](https://toppr-doubts-media.s3.amazonaws.com/images/2126244/274414aa-deba-45ea-a8ef-39b8460abe5f.jpg)
The Nernst equation the following electrochemical cell will be: Ni(s) | Ni2+ (aq)|| Ag+ (aq)| Ag A) Ecell = Eºcell-RT/F[In[Ni2+]/[Ag+12] B) Ecell = Eccl1-RT/2F[In[Ni2+1/[Ag+1?] C) Ecell = Eºcell-RT/2F[In[Ag+]2/[Ni2+]] D) Ece = Eccl1-RT/2F[In[Ni2+1/[Ag+l]
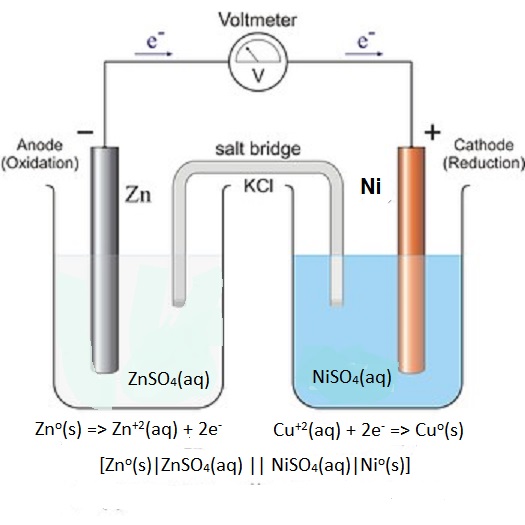
Zn(s) +Ni^(2+)(aq) -> Zn^(2+)(aq) + Ni(s) Which part of the cell conducts electrons in this reaction and describes the direction of electron flow as the cell operates? | Socratic

![PDF] Raman Spectroscopy of Nickel Sulfide Ni | Semantic Scholar PDF] Raman Spectroscopy of Nickel Sulfide Ni | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/345ef7d84582812e979cbd9a8f6545615d4ef1a4/2-Figure4-1.png)